
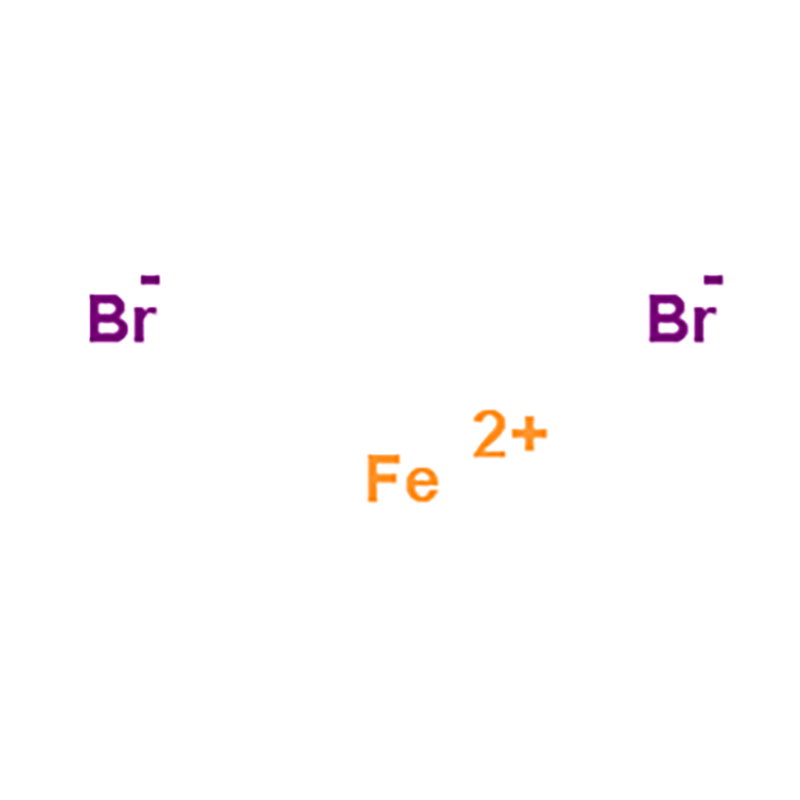


Anhydrous ferrous bromide is a bromide of iron, in which the value of the iron element is +2 chemical FeBr2, brownish yellow delirium solid, soluble in water. Light green hexahydrate was crystallized from aqueous solution at room temperature. Tetrahydrate and dihydrate can also be crystallized at higher temperature. Anhydrous salts can be obtained by heating aqueous ferrous bromide in a hydrogen bromide stream at 400 °C.
Dark reddish-brown lustrous hexagonal rhomboid plate crystals. Extremely hygroscopic. Corrosive and irritating. Soluble in water, ethanol, ether, acetic acid, slightly soluble in liquid ammonia. When heated, they sublimate and decompose gradually to form ferrous bromide and bromine. The vapor pressure of bromine is 7.331 ×103Pa at 90℃ and 1.013 ×105Pa at 139℃. Exposure to air and light will escape some of the bromine vapor. When boiling its aqueous solution, it breaks down to form ferrous bromide and bromine. Its hexahydrate is dark green crystal, soluble in its own crystalline water at 27 C. Store in low temperature sealed away from light.
Product Name: ferrous bromide Iron(II) Bromide
Molecular Formula: FeBr₂
Molecular weight: 215.65
CAS NO: 7789-46-0
Grade:anhydrous
Assay:99.99%
EINECS NO: 232-168-8
Melting point: 684 ℃(957K)
Boiling point: 934 ℃(1207K)
Density: 4.63g /cm³
Appearance: solid brownish yellow color
Used in catalytic bromination of aromatic compounds, analytical chemistry and disease medicine.
Used as catalyst for organic synthesis
Plug the container (B) with nitrogen to sweep the instrument shown in Figure ⅷ 2. When the plug is removed, a nitrogen injection can be passed through the branch pipe, thus providing an effective gas curtain to prevent the diffusion of atmospheric oxygen. 10g (0.18mol) of iron powder (hydrogen-reduced) is then placed in the container (A), followed by 100mL of methanol (which promotes evaporation and is better than water and does not require air removal) and the required 45mL of hydrobromic acid (50%). The vessel (A) is immersed in hot water in A nitrogen atmosphere to maintain A rapid rate of reaction. The reaction was completed in 2 ~ 3h. It is necessary to ensure that the hydrogen emission is completely stopped so as not to create pressure even after the instrument is turned off. Stop the nitrogen flow, and install the switch latch on the faucet nozzle to seal the instrument. The green solution was filtered through a fused glass sand filter plate into a container (B) and frozen in a dry ice ethanol freeze bath. Adjust the faucet to the open position and use an efficient mechanical pump to drain the instrument through the branch pipe. Shut off the tap and seal the instrument. Soak (A) in A dry ice freezing bath and slowly heat (B) to 100℃ so that the solvent evaporates from (B) and condenses in (A). When all the green crystalline hexamethanol complex has been converted to the white dimethanol complex (about 3h), nitrogen is introduced, the switch latch on the faucet is removed, and the collected solvent in the container (A) is emptied and discarded while the nitrogen is flowing through the branch pipe. The products in container (B) were heated at 160℃ for 4h under vacuum to complete the final solvent removal. This can be done conveniently by connecting the instrument to a mechanical pump connected in series with a suitable steam trap and by immerging the vessel (B) in an oil bath or in a small electric stove. After cooling, nitrogen is introduced and the product is moved to the reservoir. The yield was 73.7% (theoretical value 74.1%).


