-4.jpeg)
-5.jpeg)
-6.jpeg)
-4.jpeg)
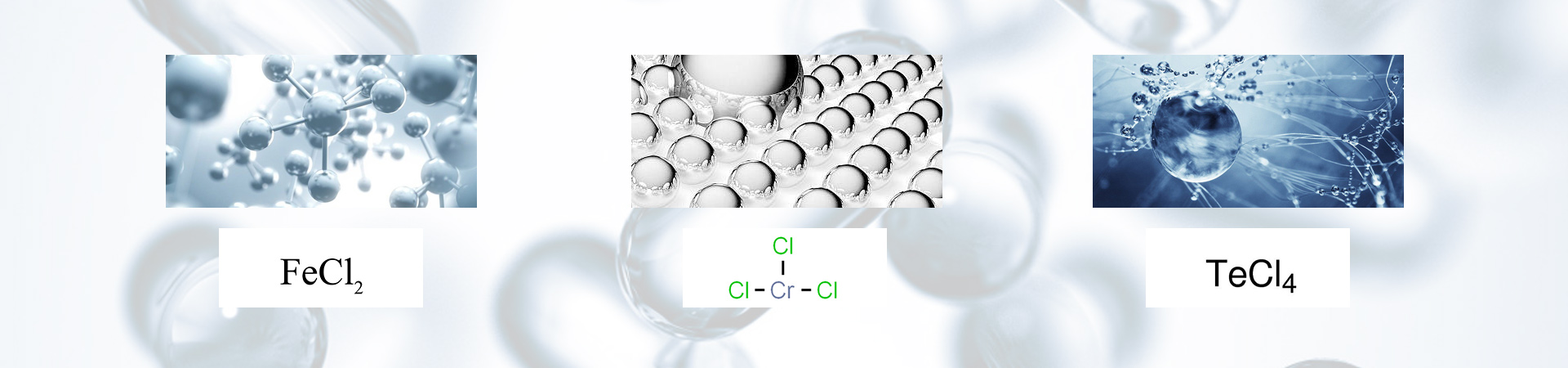
Chromium(III) chloride (also called chromic chloride) is a violet coloured solid with the formula CrCl3.
Although it is ionic, the solid state structure is kinetically inert so that anhydrous CrCl3 is surprisingly reluctant to dissolve in water. However, in the presence of a trace of a reducing agent capable of reducing Cr3+ to Cr2+, the CrCl3 dissolves rapidly to form soluble complexes containing hydrated Cr3+ ions. This inertness means that CrCl3 is generally sluggish to react without the presence of a reducing agent. When it does react it undergoes ligand substitution reactions to form other complexes of chromium(III). It reacts as a Lewis acid, forming stable chloro complexes such as [CrCl6]3-.
Chromium(III) chloride (also called chromic chloride) is a violet coloured solid with the formula CrCl3.
Although it is ionic, the solid state structure is kinetically inert so that anhydrous CrCl3 is surprisingly reluctant to dissolve in water. However, in the presence of a trace of a reducing agent capable of reducing Cr3+ to Cr2+, the CrCl3 dissolves rapidly to form soluble complexes containing hydrated Cr3+ ions. This inertness means that CrCl3 is generally sluggish to react without the presence of a reducing agent. When it does react it undergoes ligand substitution reactions to form other complexes of chromium(III). It reacts as a Lewis acid, forming stable chloro complexes such as [CrCl6]3-.
-1.jpeg)
-2.jpeg)
-3.jpg)
